Written by Joyce Smith, BS. This study demonstrates a new and innovative application of Photo Dynamic Therapy for treating circulating tumor cells.
Cancer metastasis is responsible for over 90% of all cancer deaths (1). Metastasis occurs when primary cancer cells are shed into the bloodstream, circulate through it and eventually spread to other organs where they are able to colonize. (2) These circulating tumor cells are key players in cancer metastasis.
Photodynamic Therapy is a treatment protocol that involves the use of agents called photosensitizers. When these photosensitizing agents are injected into the bloodstream, they are absorbed by cancer cells. The presence of light (laser or ultraviolet) causes the photosensitizing agent to react with oxygen and produce a chemical that is toxic and destructive to cancer cells (3, 4). While this is a safer treatment than conventional radiation and chemotherapy, it has its own disadvantages. Visible light cannot penetrate through dense tissue, particularly in the presence of blood, so its application is limited to topical areas of the body such as skin, neck, lungs, etc. (5, 6).
Researchers recently hypothesized that by removing circulating tumor cells from the blood stream, the chance of metastasis from aggressive tumors may be reduced.
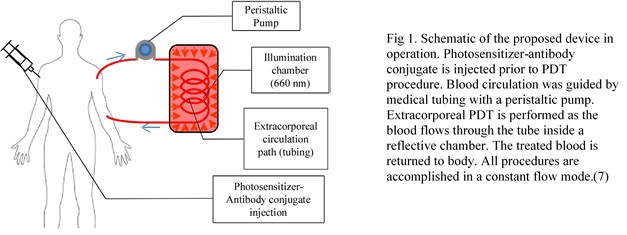
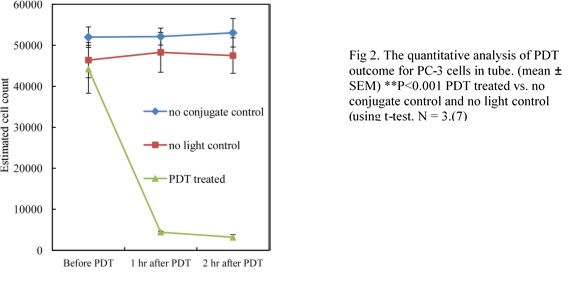
They demonstrated a new therapeutic application of photodynamic therapy (PDT) for destroying circulating tumor cells (CTCs). It involved using a photosensitizer (Ce6-CD44Ab conjugate)* that would selectively target and stain prostate cancer cells so they could be detected with a fluorescence microscope. Blood containing prostate cancer cells (PC-3) cells was treated with a photosensitizer and allowed to flow through a thin transparent medical tube only 1.02 mm in diameter (Figure 1). The tube was subjected to LED light for 2 minutes. Researchers used a fluorescence microscope to count the fluoresced cells 60 and 120 minutes after the light treatment and found a significant reduction in the amount of viable PC-3 cells. The two negative controls (one with photosensitizer and no light and the other with no photosensitizer and with light) indicated no cell death after 2 hours.
| Controls | Prostate Cancer Cells (PC-3s) | Photosensitizing Agent (Ce6-CD44Ab conjugate) | LED light |
|---|---|---|---|
| Positive Control | PC-3 cells | Photosensitizing agent | LED light |
| Negative Control | PC-3 cells | No Photosensitizing agent | LED light |
| Negative Control | PC-3 cells | Photosensitizing agent | No LED light |
The same three controls were used to demonstrate the impact of PDT on CP-3 cells that were grown in a culture media containing blood as well as in a culture media with no blood. After LED light treatment, both cultures were examined for the presence of dead cells. The researchers found that:
- The PDT on the CP-3 cells in the blood culture showed increased cell death with increasing light exposure.
- The CP-3 cells in the media that contained no blood died immediately (within 15 minutes).
- The CP-3 cells in blood media demonstrated cell death only with increasing light exposure.
- The PDT on the thin transparent tube was significantly more efficient in cell destruction than the PDT on the blood tissue culture because the presence of blood hemoglobin blocked light transmission in the blood culture, while the thin tube allowed light penetration from all angles (Figure 2).
The researchers believe “This new and innovative extracorporeal methodology of PDT suggests a practical application for treating not only circulating tumor cells but hematological pathogens as well”.
*This product is extracted from living Chlorella cells with water, 50% alcohol, dichloromethane, sodium sulfate, and acetone.
Source: Kim G, Gaitas A (2015) Extracorporeal Photo-Immunotherapy for Circulating Tumor Cells. PLoS ONE 10(5): e0127219. doi:10.1371/journal. pone.0127219
© 2015 Kim, Gaitas. Creative Commons Attribution License
Click here to read the full text study.
Posted August 4, 2015.
References:
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011; 331(6024):1559– 64.
- López‐Riquelme N, Minguela A, Villar‐Permuy F, Ciprian D, Castillejo A, Álvarez‐López MR, et al. Im- aging cytometry for counting circulating tumor cells: Comparative analysis of the CellSearch vs Image- Stream systems. Apmis. 2013; 121(12):1139–43.
- Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009; 139(7):1315–26.
- Edelman MJ, Potter P, Mahaffey KG, Frink R, Leidich RB. The potential for reintroduction of tumor cells during intraoperative blood salvage: reduction of risk with use of the RC-400 leukocyte depletion filter. Urology. 1996; 47(2):179–81.
- Doiron DR, Svaasand LO, Profio AE. Light dosimetry in tissue: application to photoradiation therapy. Porphyrin Photosensitization: Springer; 1983. p. 63–76.
- Stolik S, Delgado J, Perez A, Anasagasti L. Measurement of the penetration depths of red and near in- frared light in human “ex vivo” tissues. Journal of Photochemistry and Photobiology B: Biology. 2000; 57(2):90–3.
- Kim G, Gaitas A (2015) Extracorporeal Photo-Immunotherapy for Circulating Tumor Cells. PLos ONE 10(5): e0127219. doi:10.1371/journal.pone.