Written by Greg Arnold, DC, CSCS. 106 Chinese subjects using 750 mg stevioside in capsules had 7.6% decrease in systolic blood pressure compared to the control group.
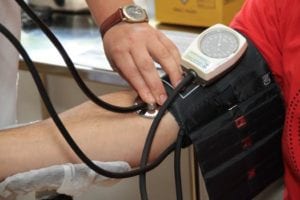 In a 2000 study, 106 Chinese subjects (53 men, 53 women) between the ages of 28 and 75 and diagnosed with high blood pressure were given either 750 milligrams of stevioside (250-milligram capsules three times daily = 60 subjects) or placebo (46 subjects) for 1 year.
In a 2000 study, 106 Chinese subjects (53 men, 53 women) between the ages of 28 and 75 and diagnosed with high blood pressure were given either 750 milligrams of stevioside (250-milligram capsules three times daily = 60 subjects) or placebo (46 subjects) for 1 year.
By the end of 1 year, stevioside decreased systolic blood pressure (the top number) by 8.4% (166.5 to 152.6 mmHg) compared to a 0.8% decrease in the placebo group (166 to 164.8 mmHg, p < 0.05). Stevioside also decreased diastolic blood pressure (the bottom number) by 13.8% (104.7 to 90.3 mmHg) compared to a 0.9% decrease in the placebo group (104.7 to 103.8 mmHg, p < 0.05). And although these results are after 1 year of supplementation, the researchers noted that blood pressure began to decrease after 7 days, statistical significance was reached after 3 months and was sustained for the remainder of the study.
In addition to the significant benefits of stevioside supplementation was the 90% compliance rate as the usage of alternative and complimentary or traditional medicine is still very common [in the Chinese population] and the adherence to traditional medicines is usually better…since stevioside is a glycoside purified from a plant, patients usually regarded it as a traditional medicine.”
For the researchers, “oral stevioside is a well-tolerated and effective modality that may be considered as an alternative or supplementary therapy for patients with hypertension.”
Source: Chan, Paul, et al. “A double‐blind placebo‐controlled study of the effectiveness and tolerability of oral stevioside in human hypertension.” British Journal of Clinical Pharmacology 50.3 (2000): 215-220.
© Blackwell Science Ltd. Br J Clin Pharmacol, 50, 215-220
Posted December 8, 2014.
Reference:
- Chan P. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br J Clin Pharmacol. Sep 2000; 50(3): 215–220





Comments (0)